The packaging world shakes hands with a plethora of different industries; Pharmaceutical, Fast Moving Consumer Goods, Food & Beverage, Cosmetics, etc. Each industry has its own unique working environment, including unique hazards that come with each. But any industry that deals with packaging shares some overlapping risks. Here are the top three compliance risks for packaging, and one potential solution that can take them all on.
The Three Monsters:
1. Recalls
Anyone involved in the packaging world knows the dreaded word “recall.” Some have had the misfortune of dealing with this problem head on, and others have only heard through the grapevine about the steep consequences that must be faced when dealing with a recall.
A recall is peculiar in that one seemingly microscopic misprint can make the difference between a successfully launched product and millions of dollars worth of logistics rollback and untold damage to quality and reputation. The consequences of recalls have short term immediate impact as well as long term ramifications, such as sanctions.
2. Process Risks
A process risk, while often less blunt than a recall, can have more subtle and extended impact, which makes them more dangerous in that they are easier to overlook. Process risks involve a failure to follow established processes. In the packaging world, an example of this could be as simple as skipping a step in the validation process for the design of a new pamphlet.

When process risks become habitual the potential for heavier risks (such as recalls) increases, productivity can decline, and the quality of output can suffer, overtime causing damage to the bottom lineand more importantly to the vital structures that keep companies afloat.
3. Illegal Practices
The risk of illegal practices is in many ways self explanatory. Everyone would like to believe that their company is immune to such risk, but illegal practices are not exclusively intentional. More often than not illegal practices take place unknowingly and are a result of the mismanagement of processes or resources.
In terms of packaging, unintentional illegal practices can take the shape of mislabelling, mismanagement, and nonobservance of legal requirements.
What causes these risks?
While there are varying factors that enhance the probability of the risks above, it is clear that the three risks share some common threads. Miscommunication is a likely cause in many errors attributed to recalls, process risks, or illegal mishaps, and can be an ill-fated result of disorganization with an individual or team, lack of transparency for the proper parties, or workflow inconsistencies. Time Pressure is another phenomenon that those involved in packaging design are intimately familiar with. The rush to get to the shelf can often mean that safekeeping processes are overlooked and corners are cut to decrease the amount of time it takes to start seeing a return on investment.

Completely eliminating these risks may not be possible, but companies can create some mitigation strategies to lower the probability of these occurrences.
One Solution to Rule Them All – Online Artwork Management Tools
While the root causes of compliance risks may seem intimidating at a glance, the good news is that these issues can be prevented with proper support. In today’s technological age there is a wide range of Artwork Management Systems (AMS) that boost communication efficiency and workflow organization through a plethora of verification, workflow management, and proofing tools. By using an AMS, companies are able to support the artwork design process by eliminating old manual processes and lengthy back and forth email exchanges with different parties.
But what really makes an AMS so interesting for organizations? You can find some of the well-known benefits below.
Organize communication and create one source of truth
- Create one centralized artwork repository containing all versions, which is accessible at any time.
- Eliminate back and forth email exchanges and communicate about the project within the project itself.
- Allow for internal and external feedback and approvals, tracking status, comments and confirmations to be accessed in one single place.
- Record full audit trails of what happens with the project from briefing to print.
Increase workflow visibility
- Identify & eliminate bottlenecks in your workflow process, see where improvements are needed most.
- Track KPIs & gain actionable insights, visualize your workflow and prevent process errors.
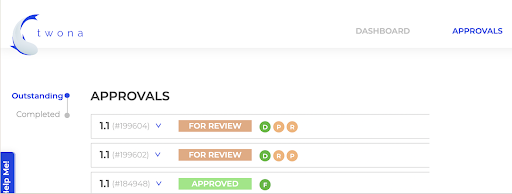
Streamline approvals
- Share files of any size, no more inbox limits.
- Request feedback approvals from internal and external stakeholders on the right version.
- Return annotated files and attach new ones for changing purposes.
- Visualize all approvals from one single place.
Give you the power of proofing
- Gain a clear view of changes in packaging done through different versions (text & graphic) and reduce the need to inspect manually.
- Red line overlapping documents and also x-ray side by-side comparisons.
- Download proofing reports.
- Comment directly on documents, where multi-user notes combine together.
Automate and integrate to eliminate redundant tasks
- Set automatic notifications.
- Integrate with other tools to speed up processes.
Do the reasons above sound like something that would make your process easier, and give you the peace of mind that your company requires? If the answer is yes, you may be a good fit for an AMS.
Compliance Risks are Nothing to Fear
Compliance risks are an ever present obstacle that anyone in the packaging industry must stay vigilant against. But that doesn’t mean that they are insurmountable. With proper workflow transparency and consistency, compliance risks are easily mitigated. If you’re ever in fear, you can depend on AMS and other solutions to save the day.
Published by Twona: Twona is a multi-faceted packaging specialist company with 20 years of experience in software solutions and packaging design. Twona’s flagship product Twona AMS has helped companies worldwide organize and streamline their packaging design process. You can start a free trial of Twona AMS today by clicking the following link , or request a demo now.